Minerals are the constituents that form rocks, the solid fabric of our planet. Among the thousands of known minerals, silicate minerals stand out as the most abundant and geologically significant. If the Earth had a recipe, silicon and oxygen would be its key ingredients. These two elements combine in countless ways to form silicate minerals, which make up roughly 90% of the Earth’s crust.
In this article, we’ll dive into the fascinating world of silicate minerals. We’ll explore their structure, classification, and how they play a critical role in shaping the Earth as we know it, all told in a way that connects geology with the human story of curiosity and discovery.
Why Silicates Matter?
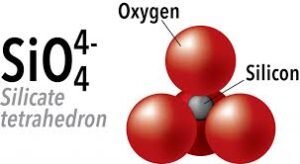
Silicate minerals are built from a basic structural unit: the silica tetrahedron. This is a four-sided pyramid-shaped molecule composed of one silicon atom bonded to four oxygen atoms (SiO₄). What makes silicates special is how these tetrahedra join together, like Lego blocks forming everything from single chains to complex three-dimensional frameworks.
These structures influence not just the mineral’s appearance but also its hardness, melting point, and how it weathers over time. So, let’s explore the main types of silicate minerals, grouped by how their silica tetrahedra are arranged.
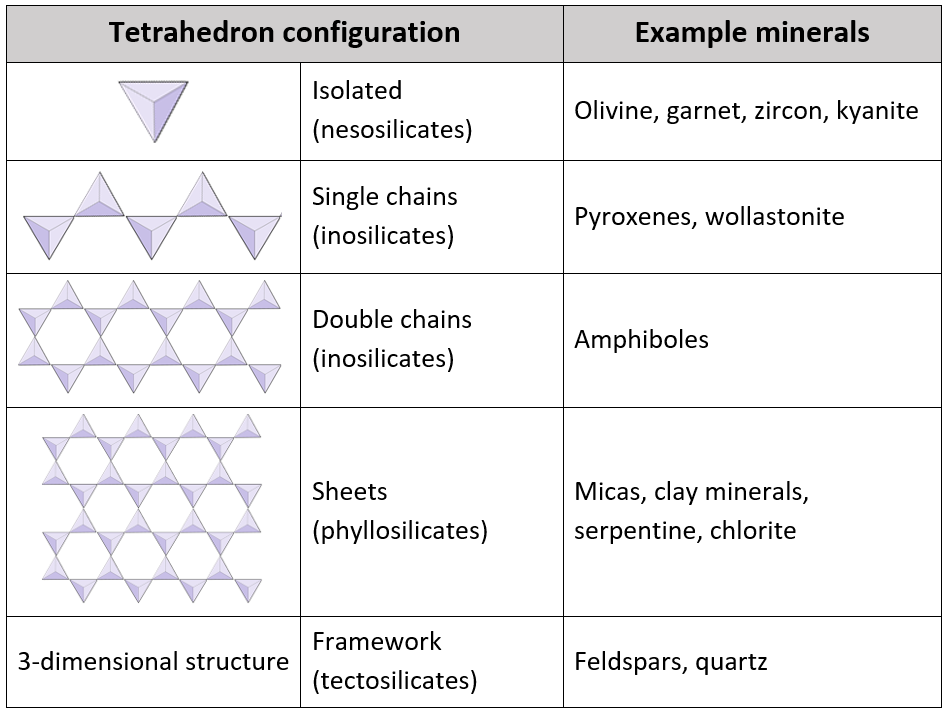
Nesosilicates (Isolated Tetrahedra)
In nesosilicates, each silica tetrahedron stands alone, not sharing any oxygen atoms with neighboring tetrahedra. This isolated structure results in strong bonds and relatively dense, hard minerals.
Common Examples:
- Olivine: A major component of the Earth’s mantle, often green, found in basalt and peridotite.
- Garnet: Known for its use as a gemstone and in metamorphic rocks.
- Zircon: Famous for its ability to preserve ancient geological ages through radiometric dating.
These minerals often form in high-temperature environments, such as the mantle or during regional metamorphism. When you hold a garnet or piece of olivine, you’re holding a fragment of Earth’s inner engine.
Sorosilicates (Double Tetrahedra)
Sorosilicates are a bit rarer and consist of two silica tetrahedra joined at one oxygen, forming Si₂O₇ groups.
Common Example:
- Epidote: Often green and found in metamorphic rocks.
Though not as widespread, sorosilicates provide insight into low-grade metamorphic conditions and hydrothermal alteration zones.
Cyclosilicates (Ring Silicates)
In cyclosilicates, the silica tetrahedra are linked in rings, most commonly six-membered rings (Si₆O₁₈), though three- and four-membered rings exist too. This ring structure gives rise to interesting symmetry and beautiful crystals.
Common Examples:
- Beryl: Includes gemstones like emerald and aquamarine.
- Tourmaline: A colorful and complex mineral found in igneous and metamorphic rocks.
These minerals often form in pegmatites, those extraordinary veins of large crystals that geologists hunt for in granitic terrains.
Inosilicates (Chain Silicates)
Inosilicates are split into single-chain and double-chain structures.
Single-Chain Inosilicates:
Here, each tetrahedron shares two oxygen atoms with its neighbors, forming a continuous chain.
- Example: Pyroxenes (like augite, diopside).
- Geological Context: Found in basalt, gabbro, and peridotite—key players in igneous petrology.
Double-Chain Inosilicates:
In this group, tetrahedra share two or three oxygen atoms, forming double chains.
- Example: Amphiboles (like hornblende, tremolite).
- Geological Context: Common in metamorphic rocks like schist and amphibolite.
These minerals are important for interpreting temperature, pressure, and fluid presence during rock formation.
Phyllosilicates (Sheet Silicates)
In phyllosilicates, each silica tetrahedron shares three oxygen atoms, creating wide, flat sheets. These sheets are often weakly bonded together, allowing for easy splitting—a property called cleavage.
Common Examples:
- Mica Group: Biotite (dark) and muscovite (light), both flaky and shiny.
- Chlorite: Green mineral found in low-grade metamorphic rocks.
- Clay minerals: Such as kaolinite and montmorillonite, are crucial in soil science.
These minerals are soft, flexible, and slippery, making them vital not just in geology but in ceramics, drilling mud, and cosmetics.
Tectosilicates (Framework Silicates)
The most complex and abundant group, tectosilicates, involves all four oxygen atoms in each tetrahedron being shared with neighboring tetrahedra. This creates an incredibly stable and interconnected three-dimensional framework.
Common Examples:
- Quartz: Pure silica, found in almost all rock types.
- Feldspar Group:
- Plagioclase: Common in basalt and granite.
- Orthoclase: Found in granite and pegmatites.
- Zeolites: Microporous minerals used in water purification and gas separation.
Tectosilicates form the backbone of continental crust, especially through feldspars. They’re not just beautiful but geologically fundamental.
Understanding through observation
One of the joys of geology is that you don’t need a lab to see the difference between silicate groups. Take a granite rock, for instance:
- Quartz looks glassy and irregular.
- Feldspar is blocky and often pink or white.
- Mica flakes off in thin, shiny sheets.
These minerals tell a story about how the granite cooled, what it’s made of, and what tectonic processes might have created it. With time and practice, recognizing silicate types in hand samples or thin sections becomes a window into Earth’s inner workings.
Why does silicate classification matter?
Understanding silicate mineral types isn’t just academic—it’s practically important. Here’s why:
- Resource exploration: Many ores form in or alongside specific silicate minerals.
- Soil and agriculture: Clay minerals control nutrient retention and water drainage.
- Environmental geology: Certain silicates like zeolites can absorb toxins.
- Planetary science: Silicate mineralogy helps us compare Earth with other planetary bodies.
From building materials to microchips, the reach of silicates goes far beyond field geology.
Practice Questions:
Q1: What is the basic building block of all silicate minerals?
A1: The fundamental building block of all silicate minerals is the silicon-oxygen tetrahedron (SiO₄⁴⁻). It consists of one silicon atom centrally bonded to four oxygen atoms arranged in a tetrahedral shape.
Q2: How are silicate structures classified?
A2: Silicate structures are classified based on how the SiO₄ tetrahedra are linked together. The major types include isolated tetrahedra, single chains, double chains, sheets, and three-dimensional frameworks.
Q3: What are isolated tetrahedra (nesosilicates)?
A3: In nesosilicates, such as olivine, the SiO₄ tetrahedra do not share any oxygen atoms with adjacent tetrahedra. They are bonded by interstitial cations like Mg²⁺ or Fe²⁺.
Q4: What are single-chain silicates?
A4: Single-chain silicates, like pyroxenes, have tetrahedra linked in a linear chain by sharing two oxygen atoms. This results in a more complex, elongated structure.
Q5: What are double-chain silicates?
A5: In double-chain silicates such as amphiboles, two single chains are bonded together by sharing oxygen atoms, forming a more open and less dense structure than pyroxenes.
Q6: What are sheet silicates?
A6: Sheet silicates, or phyllosilicates, like mica and clay minerals, have tetrahedra connected in flat, two-dimensional sheets. These sheets are bonded together by weaker forces, allowing easy cleavage.
Q7: What are framework silicates?
A7: Framework silicates, or tectosilicates, like quartz and feldspar, have all four oxygen atoms of each tetrahedron shared with adjacent tetrahedra, creating a strong and rigid three-dimensional network.
Q8: How does silicate structure affect magma viscosity?
A8: Magmas with more complex silicate structures (like sheet or framework silicates) have higher viscosity due to stronger polymerization, while those with simpler structures (like isolated tetrahedra) are less viscous and flow more easily.
Q9: Why are silicates the most abundant group of minerals in the crust?
A9: Silicates dominate Earth’s crust because silicon and oxygen are the most abundant elements in the crust, naturally forming a wide range of minerals with different structural complexities and stability conditions.


Very interesting topic, thankyou for posting. “He who seizes the right moment is the right man.” by Johann Wolfgang von Goethe.